12+ Chapter 7 Ionic And Metallic Bonding
We will learn about them in detail below. Web A metal ion in aqueous solution or aqua ion is a cation dissolved in water of chemical formula MH 2 O n zThe solvation number n determined by a variety of experimental methods is 4 for Li and Be 2 and 6 for most elements in periods 3 and 4 of the periodic table.

Ionic Compounds Ionic Bonds Properties Formation Examples Videos
Bonding between a metal and a nonmetal is often ionic.

. Web NCERT Solutions for Class 10 Maths Chapter 12. Lanthanide and actinide aqua ions have higher solvation numbers often 8 to 9. Web Join an activity with your class and find or create your own quizzes and flashcards.
The ionic bonded molecules in their aqueous solutions or in the molten state are good conductors of electricity. If relatively little ionization occurs the acid or base is weakAs will be evident throughout the remainder of this chapter there are many more weak. Chemically gold is a transition metal and a group 11 elementIt is one of the least reactive chemical.
If the ionization reaction is essentially complete the acid or base is termed strong. This uses 12 e-. The categories are decided based on what elements the hydrogen forms bonds with or simply on the basis of chemical bonding.
The empty string is the special case where the sequence has length zero so there are no symbols in the string. Most of the rocks and minerals that make up the Earths crust are composed of positive and negative ions held together by ionic bonding. Web 33 Ionic Bonding.
NCERT Solutions for Class 10 Maths Chapter 13. You are very familiar with some ionic compounds such as sodium chloride NaCl. The ionic bonded molecules have high melting and boiling point.
Web Important Questions for Class 12 Chemistry Chapter 8 The d- and f-Block Elements Class 12 Important Questions. Ionic bonding is a sort of chemical bonding that occurs when electrons are transferred from one atom or molecule to another. The three types of hydrides are ionic covalent and metallic hydrides.
Web Download The Solid State CBSE Class 12 Chemistry Chapter 1 notes PDF for free. Ionic bond with molecular formula ZY 2. The ionic bond has charge separation and so they are the most reactive of all the bonds in the proper medium.
Ionic compounds with almost. If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution. Aurum and atomic number 79.
One merely needs to identify all the ions present in the solution and then consider if possible cationanion pairing could result in an insoluble compound. The relative strength of an acid or base is the extent to which it ionizes when dissolved in water. Predict the charge of common metallic and nonmetallic elements and write their.
Web ionic compounds cations anions metallic bonding octet rule. Write the balanced ionic equation for the reaction between ferrous sulphate and acidified potassium permanganate solution. Solutions are all around us.
Lesson 20 - Metallic Bonding. Section you will be able to. Web The solubility guidelines in Table 71 may be used to predict whether a precipitation reaction will occur when solutions of soluble ionic compounds are mixed together.
I forms an anion ii forms a cation b State the type of bond between Y and Z and give its molecular formula. Web Abrupt and controllable changes of optical properties are achieved by engineering the interaction between light and an array of optical scatterers called optical antennas 18 19 which can take a variety of forms including metallic or dielectric micronano-particles 20 21 apertures formed in metallic films 22 23 and their multi. Secure good marks by referring NCERT Class 12 The Solid State revision notes prepared by Vedantu experts.
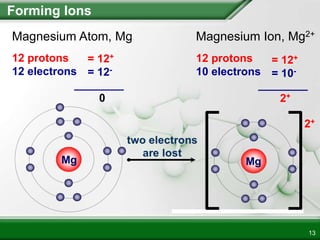
In addition to metallic bonding there is extra covalent bonding due to presence of. An ionic compound is an electrically neutral compound consisting of positive and negative ions. An ionic bond is the electrostatic attraction formed between cations and - anions.
Metallic bonding textFe Cu textAg Mg. Web Acid and Base Ionization Constants. PubMed Journals helped people follow the latest biomedical literature by making it easier to find and follow journals browse new articles and included a Journal News Feed to track new arrivals news links trending articles and important article updates.
A Elements X Y and Z have atomic numbers 6 9 and 12 respectively. Chemical Bonding is Chapter 4 of Class 11 Chemistry. Taking MgCl as an example of electrovalent bond CCl 4 as a covalent.
The atoms in polyatomic ions such as OH NO 3 NO 3 and NH 4 NH 4 are held together by polar covalent bonds. With some exceptions those in nonmetals are fixed in place resulting in nonmetals. Work Energy Power and Thermodynamics.
Web The ionic bonds are the strongest of all the bonds. Formally a string is a finite ordered sequence of characters such as letters digits or spaces. Web Lesson 7 - Ionic Compounds.
Chapter 12 Practice Test Practice test. This makes it one of the higher atomic number elements that occur naturally. Web Bonds between two nonmetals are generally covalent.
Resistivity is commonly represented by the Greek letter ρ The SI unit of electrical resistivity is the ohm-meter. 12012021 Table of Contents. Web Almost two years ago we launched PubMed Journals an NCBI Labs project.
Some compounds contain both covalent and ionic bonds. Web Gold is a chemical element with the symbol Au from Latin. They range from colorless gases like hydrogen to shiny solids like carbon as graphiteThe electrons in nonmetals behave differently from those in metals.
73 Lewis Symbols and. It is a bright slightly orange-yellow dense soft malleable and ductile metal in a pure form. Explain the formation of cations anions and ionic compounds.
Air for example is a solution. So far we have used 4 12 16 electrons. The type of bond formed depends on the ionization energy and electronegativity of the atoms involved.
This is unique to metallic bonds. This leaves us with 4 e- left over. Ionic bonds a type of bond between a metal and a non-metal are formed when the metal ion gives some of its outermost.
One atom loses an electron in this process which is then gained by another atom. Ferrum and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic tableIt is by mass the most common element on Earth right in front of oxygen 321 and 301 respectively forming much of Earths outer and inner coreIt is the fourth most.
Web Iron ˈ aɪ ə n is a chemical element with symbol Fe from Latin. Web Electrical resistivity also called specific electrical resistance or volume resistivity is a fundamental property of a material that measures how strongly it resists electric currentA low resistivity indicates a material that readily allows electric current. Web In chemistry a nonmetal is a chemical element that generally lacks a predominance of metallic properties.
Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. Web 71 Ionic Bonding. The density of the substance is reduced as a result of this flaw.
The main idea behind this chapter is the.

Chapter 7 Ionic And Metallic Bonding 7 2 Ionic Bonds And Ppt Video Online Download
Properties And Formation Of Ionic Compounds Powerpoint
Ppt Unit 5 Chemical Bonding Overview Ionic Compounds Powerpoint Presentation Id 907556

Chapter 7 Ionic And Metallic Bonding 7 2 Ionic Bonds And Ppt Video Online Download

Ionic Or Electrovalent Compounds Properties Characteristics With Videos

Chapter 7 Ionic And Metallic Bonding Ppt Video Online Download

Chapter 7 Ionic And Metallic Bonding Ppt Download

Chapter 7 Ionic And Metallic Bonding 7 2 Ionic Bonds And Ppt Download

Ionic Compounds Ionic Bonds Properties Formation Examples Videos

Ch 7 Ionic And Metallic Bonding Youtube

Coordination Compounds Class 12 Notes Chemistry Chapter 9 Samar Education

Ppt Unit 5 Chemical Bonding Overview Ionic Compounds Powerpoint Presentation Id 907556
Pdf 7 Transition Metal Chemistry

Ionic And Metallic Bonding Pdf Free Download

Atomically Precise Clusters Of Noble Metals Emerging Link Between Atoms And Nanoparticles Chemical Reviews

Chemical Bonds Solutions Examples Worksheets Videos Games Activities

Chapter 7 Ionic And Metallic Bonding 7 3 Bonding In Metals 7 1 Ions Ppt Download